How India’s vaccine drive turned out badly
It required 31-year-old Sneha Marathe a large portion of a day to book an arrangement online for a Covid immunization.
“It was a round of ‘quickest finger first’,” she says. “The openings topped off in three seconds.” But the hospital dropped her space without a second to spare: they had no immunizations. Ms Marathe returned to pursue another arrangement.
Every one of the long term olds in India need to enroll on the public authority’s CoWin stage to get immunized. With interest for hits far overwhelming inventory, educated Indians are in any event, composing code to corner tricky arrangements.
Ms Marathe can’t code, yet she is among a large number of Indians who are on the correct side of the country’s advanced gap – not at all like a huge number of other people who don’t approach cell phones or the web, right now the solitary course to a hit.
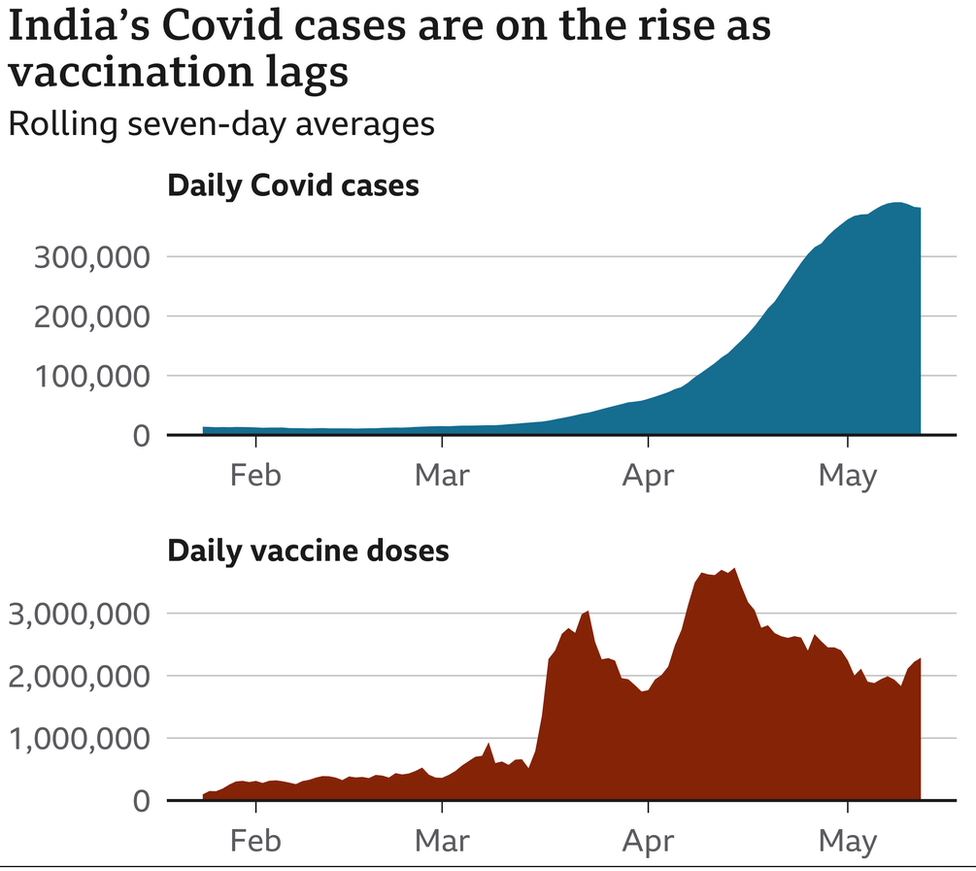
Leader Narendra Modi’s government has opened up inoculations for approximately 960 million qualified Indians without having anything near the necessary inventory – more than 1.8 billion portions.
More regrettable, the serious deficiency comes in the midst of a dangerous second Covid wave and admonitions of a looming third wave.
A mixed drink of bumbles – lack of foresight, piecemeal securing and unregulated valuing – by Mr Modi’s administration has moved India’s antibody head toward a profoundly baseless rivalry, general wellbeing specialists told the BBC.
How did the world’s biggest immunization producer, frequently named the “drug store of the world” for conventional medications, end up with scarcely any antibodies for itself?
A piecemeal system
“India held up till January to put orders for its immunizations when it might have pre-requested them a whole lot sooner. Also, it obtained such unimportant sums,” says Achal Prabhala, a co-ordinator with AccessIBSA, which lobbies for admittance to medications in India, Brazil and South Africa.
Among January and May 2021, India purchased around 350 million dosages of the two endorsed antibodies – the Oxford-AstraZeneca punch, fabricated as Covishield by the Serum Institute of India (SII), and Covaxin by Indian firm Bharat Biotech. At $2 per portion, they were among the least expensive on the planet, however not almost enough to immunize even 20% of the nation’s populace.
Announcing that India had crushed Covid, Mr Modi even took to “immunization strategy”, sending out a larger number of punches than were managed in India by March.
Differentiation that with the US or EU, who pre-requested a larger number of portions than they required almost a year prior to the antibodies opened up for inoculation.
“This ensured antibody producers a market, gave them conviction to gauge supply and deals, and guaranteed that a portion of these legislatures got enormous amounts as fast as could really be expected, when the immunizations were prepared,” Mr Prabhala says.
In contrast to the US and the UK, India likewise held up until 20 April – well into the subsequent wave – to stretch out a $610m financing line to SII and Bharat Biotech to support creation.
Another disappointment, as indicated by Malini Aisola, co-convener of the All India Drug Action Network, was the choice not to enroll the tremendous wrap of India’s assembling abilities – biologics plants, for example, that might have been repurposed into immunization creation lines.
Once more, four firms, including three government-claimed ones, have as of late been offered rights to make Covaxin, which is halfway freely supported.
Then again, by early April, Russian engineers of Sputnik V, had inked fabricating manages a large group of Indian pharma organizations, which are set to create the immunization.
A broke market
As the sole purchaser at first, the central government might have held far more prominent influence over evaluating, Ms Aisola says.
“Brought together mass obtainment would have permitted the cost to descend from $2. Rather it has gone up,” she adds.
This is on the grounds that since 1 May, it has been up to singular states and private hospitals to facilitate their own arrangements with producers.
Resistance groups have considered it a “trick”, saying the government had relinquished its obligation, opening up “crippling rivalry among states”.
States need to pay twofold – $4 – the central government’s rate for a portion of Covishield and four fold the amount of for Covaxin – $8. This was after the two organizations brought down costs for states as a “generous motion”. States are additionally vieing for scant stocks close by private hospitals, which can give the expenses for clients.
The outcome: an authentic unrestricted economy for immunizations that have been created and made with both public and private subsidizing. At private hospitals, a solitary portion would now be able to cost up to 1,500 rupees ($20; £14).
A few states have now reported designs to import different antibodies from Pfizer, Moderna and Johnson and Johnson. However, no producer can ensure supply in the following not many months since more extravagant nations have pre-requested stocks.
Sputnik V has been endorsed, however it’s as yet muddled when the immunizations will be carried out.
Should India’s antibodies be so expensive?
Some have blamed SII and Bharat Biotech for “exploitative” during a pandemic, particularly in the wake of getting public subsidizing.
In any case, others say they faced generous challenges and that the flaw lies with the public authority. India is the solitary country where the government isn’t the sole purchaser, and one of only a handful not many where immunizations are not free.
Yet, general wellbeing specialists concur that SII and Bharat Biotech should be more straightforward about their assembling costs and their business contracts.
Ms Aisola says SII needs to unveil how it spent the $300m it got from the global Covax plot and the Gates Foundation, subsidizing which was intended to fund immunizations for low-pay nations. SII has neglected to do as such, somewhat on the grounds that India prohibited fares. The organization is likewise handling a legitimate notification from AstraZeneca for defaulting on its guarantee to send half of its stockpile to low-pay nations.
General wellbeing specialists are likewise calling for examination of the Indian government’s agreement with Bharat Biotech, particularly since the Indian Council of Medical Research has said it “shares” licensed innovation (IP) for Covaxin, which it created alongside the organization. Be that as it may, the hit costs more – frequently twofold – than Covishield.
“They say they share IP yet what kind of an understanding did they sign? Does it give them [the government] the option to supersede any statements if there should arise an occurrence of a crisis?” asks Dr Anant Bhan, a general wellbeing master.
While India has upheld deferring the licenses on unfamiliar made antibodies, it has taken no action to suspend it for Covaxin.
In spite of its worldwide position, it has gone against ideas from resistance pioneers to conjure mandatory authorizing and permit other pharma organizations to fabricate the endorsed immunizations, saying these actions would demonstrate “counterproductive”.
Dr Bhan concurs that at this stage it would require some investment to move innovation and assemble limit in other pharma organizations – yet he likewise says it’s hazy why none of this was endeavored before.
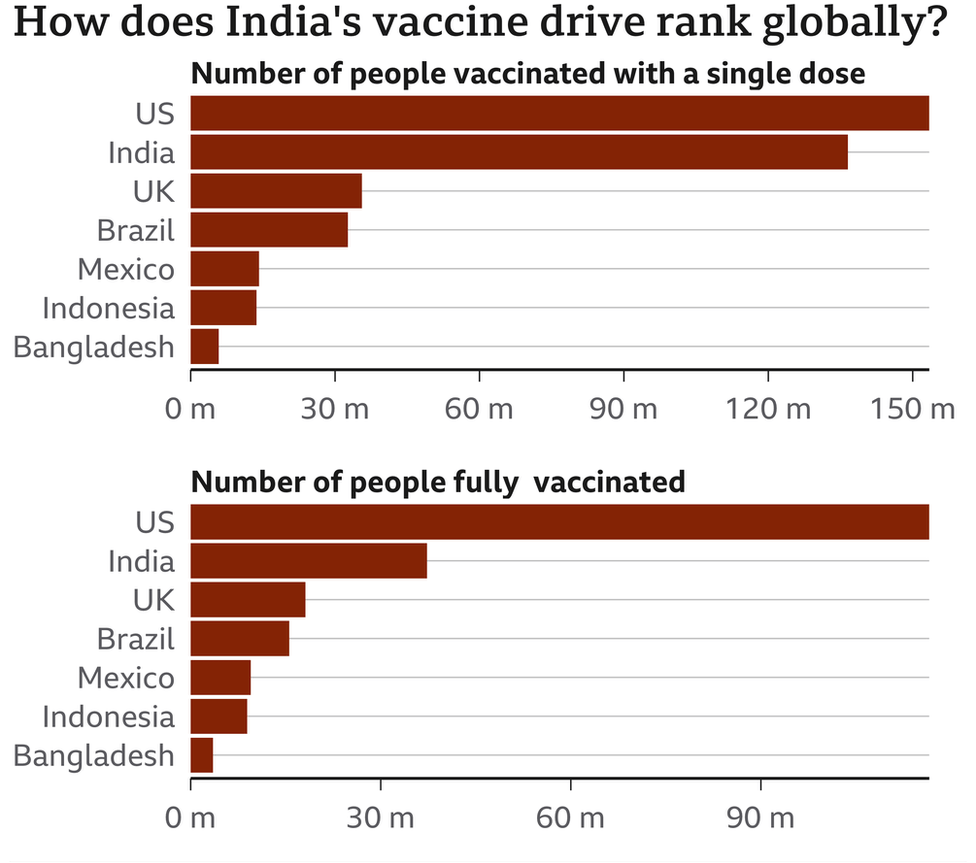
Diagram showing how India’s immunization drive positions internationally.
Immunizing even 70% of India’s 1.4 billion individuals was continually going to be a long exercise in arranging and tolerance. Yet, given the country’s solid record on inoculation, it was anything but an unthinkable assignment, Dr Bhan says.
Notwithstanding, why the public authority decided to depend on only two organizations who would now be able to control supply and direct costs is an inquiry that couple of have answers to.





